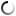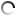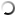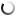It is generally accepted that oxygen is slightly soluble in copper in the solid state. When copper melt solidifies, oxygen is released as Cu-Cu2O eutectic. Oxygen is a harmful impurity since in case of its large content, electrical and thermal conductivity, plasticity and corrosive properties are sufficiently reduced. The characteristics of soldering, welding and tinning also deteriorate.
Oxygen has a negative effect on copper process properties; in particular, copper containing more than 0.1% of oxygen is disposed to destruction during hot forming operation.
Hydrogen is much soluble in both solid and liquid copper. The solubility of hydrogen in copper during melting sharply increases from 4 to 12 cm³ per 100 g of copper.
Especially, hydrogen has a negative effect on copper containing oxygen. Such copper upon heating loses its plasticity, and becomes brittle and cracked. The essence of this phenomenon lies in the fact that hydrogen reacts with the oxygen of copper oxide at high temperatures, and at this, large volumes of water vapor are generated, the pressure increases, cracks are formed, and copper material is broken.
It should also be noted that the solubility of hydrogen in copper depends not only on the temperature but also on the oxygen content: the more oxygen in liquid copper, the less hydrogen.
Technologically, it is necessary to leave only a low necessary (optimal) amount of oxygen in liquid copper so that dissolved oxygen reacts with hydrogen to generate water vapors released from the melt during metal solidification. By oxidizing hydrogen, a decrease in dissolved hydrogen content is achieved in the copper melt. Then, using deoxidizing agents, the oxygen content is reduced in the copper melt. At this, the most important is the choice of a deoxidizing agent that will not deteriorate the electrical and thermal conductivity of copper.
Use of Сu-B (2%) addition alloy is a traditional way to improve the quality of copper alloys.
Boron is an effective deoxidizer for copper, while it has no negative effect on electrical conductivity. This element is much more effective than other deoxidizers such as phosphorus, lithium, and magnesium. Table 1 shows the comparative analysis of the efficiency of deoxidizers.
Deoxidizer
Boron
Phosphorus
Lithium
Magnesium
Calcium
Zirconium
Rate, kg per oxygen kg
0.45
0.77
0.86
1.52
2.50
2.85
Ratio
1.0
1.7
1.9
3.4
5.6
6.3
In copper alloys, the introduction of boron also reduces a grain size, inhibits the growth of grains upon heating, and as a result improves mechanical properties, in particular, plasticity.
Use of boron-containing tablet preparation (flux) as a deoxidizer as well as the introduction of Cu-B addition alloy effectively reduces the oxygen content. The preparation is used for pure copper, brass and bronze melts.
As opposed to copper-phosphorus addition alloy, the preparation does not deteriorate the electrical and thermal conductivity of copper. Reduction of cast material grain is also observed. The preparation is supplied in the form of 0.2-kg tablets, the recommended rate is 0.05-0.1% by weight.
The microstructures of copper rod samples with an 8.0-mm diameter obtained by continuous crystallization from pure copper melt of M1 grade at one of the enterprises of Ukraine are stated below.
The analysis of microstructures shows a significant decrease in the oxygen content in the samples after using a boron-containing preparation with a 0.05% amount of the charge mass. This result is confirmed by direct measurement of the oxygen content in wire rod samples before and after treatment: 0.035% before treatment and 0.022% after treatment.
Eutectic colonies are located along a heat sink line.
According to the conclusion of the specialists of the enterprise using this technology, the mechanical properties of wire rod after applying the boron-containing preparation are much better. First of all, plasticity is higher, which enables to produce wires with a smaller cross-section.
Thus, based on the metallographic data of the structure of the cast material of M1 copper rod grade before and after treatment of liquid copper with the boron-containing preparation, an unambiguous conclusion can be drawn about the positive effect of boron on copper properties.
Based on more than 30 years of experience in working with non-ferrous metal melts, our specialists can offer “Customer” the optimum process chain for the production of high-quality copper alloys.

Fig. 1.1

Fig. 1.2
Fig.1.1-1.2. Microstructure of M1 copper grade, continuous casting, crystallizer, a rod diameter is 8.0 mm before processing. The oxygen content is 0.035% (mass). Magnification 200х.

Fig. 2.1

Fig. 2.2
Fig. 2.1-2.2. Microstructure of M1 copper grade, continuous casting, crystallizer, a rod diameter is 8.0 mm after processing with the boron-containing preparation. The oxygen content is 0.022% (mass). Magnification 200х.
Авторы: Сезоненко Ю.Д. ннж., Сезоненко А.Ю. к.ф.-м.н, Односум В.В. к.ф.-м.н.